Typical intuition from traditional science makes it difficult to understand how such randomness could possibly arise. But the discovery in this book that a wide range of systems can generate randomness even with very simple initial conditions makes it seem considerably less surprising.
But what about reversibility? The underlying rules for the cellular automaton used in the picture below are precisely reversible. Yet the picture itself does not at first appear to be at all reversible. For there appears to be an irreversible increase in randomness as one goes down successive panels on the page.
The resolution of this apparent conflict is however fairly straightforward. For as the picture on the facing page demonstrates, if the
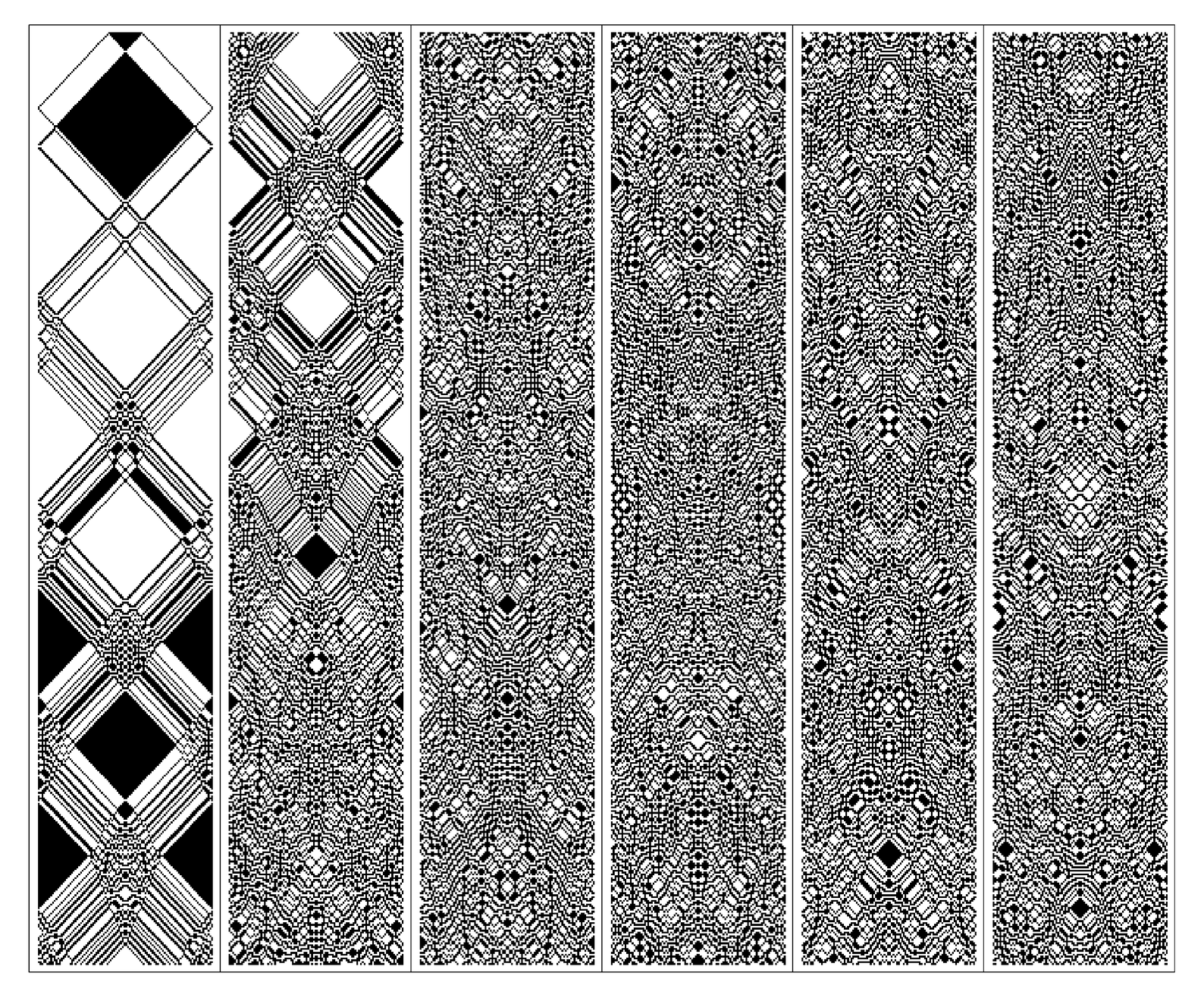
A reversible cellular automaton that exhibits seemingly irreversible behavior. Starting from an initial condition in which all black cells or particles lie at the center of a box, the distribution becomes progressively more random. Such behavior appears to be the central phenomenon responsible for the Second Law of Thermodynamics. The specific cellular automaton used here is rule 122R. The system is restricted to a region of size 100 cells.